GCG Liquid Biopsy
Pan cancer test from a single simple blood draw!

GCG-Oncomine Pan-cancer (LBx) Analysis Services is a non-hereditary solid cancer NGS panel test (liquid biopsy) that examines DNA released from tumor cells (circulating tumor DNA, ctDNA) found in the peripheral blood of patients with malignant solid carcinoma.
It is a laboratory developed test (LDT) of GC Genome utilizing the Oncomine Pan cancer Cell-free Assay(RUO) from Thermo fisher.
What is Liquid Biopsy and Why do we Need it?
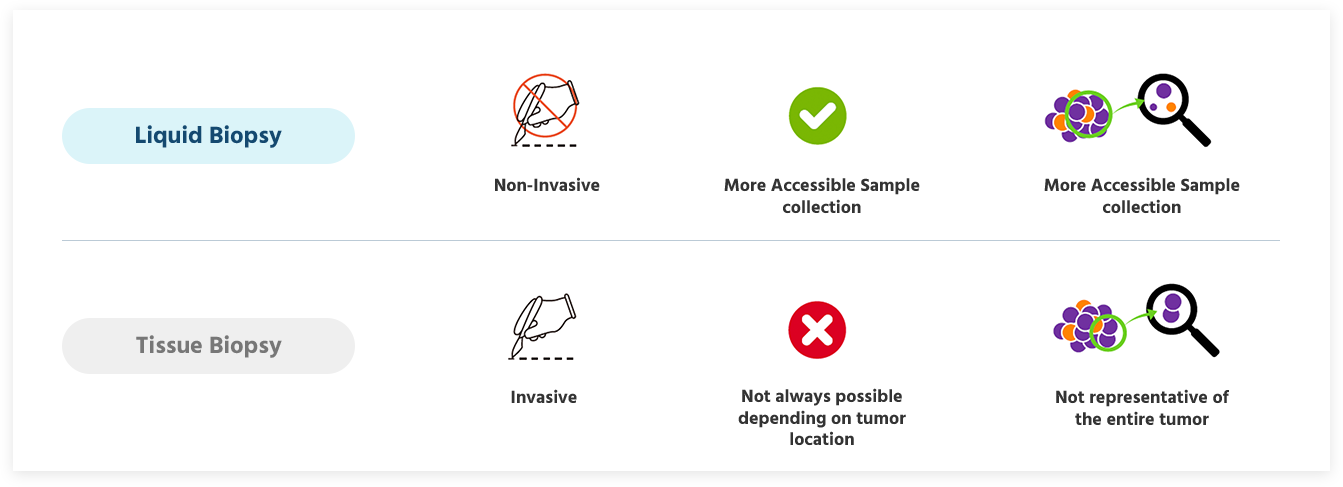
When it comes to diagnosing cancer, traditional tissue biopsy involves invasive procedures to extract tissue samples. Nevertheless, this approach may not always be possible, depending on the tumor location and the condition of the patients.
Moreover, the insights gathered from tissue biopsy only reflect the biological traits of the sampled tumor tissue. So, in some cases this information cannot represent the entire tumor.
Liquid Biopsy vs Tissue biopsy
Liquid Biopsy is a technology that can detect various genetic changes in cancer cells using the circulating tumor DNA (ctDNA) or circulating tumor cell (CTC), exosome, microRNA, tumor educated platelets. With the right sensitivity and specificity, these can be used for diagnosis, treatment decisions and monitoring of cancers. Among them, the first to be applied clinically was liquid biopsy using ctDNA from the peripheral blood.
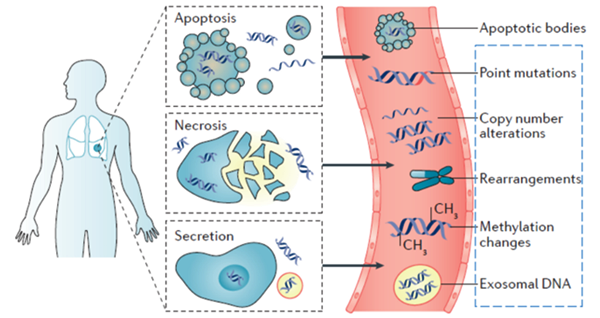
Terms to Understand:Cell-Free DNA (cfDNA) & Circulating Tumor DNA (ctDNA)
cfDNA (cell-free DNA) represents small pieces of DNA floating outside of the cells in various body fluids including blood. In healthy individuals, cfDNA exists in the bloodstream at concentrations ranging from 1 to 10 ng/ml. It plays a role in various physiological and pathological processes, including immunity, blood clotting, aging, and cancer.
In the case of cancer patients, a portion of cfDNA originates from tumors. This tumor-derived DNA is referred to as ctDNA (circulating tumor DNA), and it carries the same genetic mutations found in the primary tumor cells. Typically, ctDNA is released when tumor cells die and can be derived from both circulating tumor cells and viable tumor cells.
Why should you consider liquid biopsy?
Liquid biopsy is a non-invasive procedure that requires only a simple blood draw or collection of other body fluids, eliminating the need for invasive surgery or tissue extraction.
Additionally, Liquid biopsy can identify circulating tumor cells and cell-free DNA shed by tumors.
Lastly, liquid biopsy enables the sampling of multiple time points, allowing for real-time monitoring of disease progression and treatment response. It provides a dynamic view of the tumor heterogeneity, as it captures genetic changes from different tumor sites within the body.
What is Liquid Biopsy and Why do we Need it?
When it comes to diagnosing cancer, traditional tissue biopsy involves invasive procedures to extract tissue samples. Nevertheless, this approach may not always be possible, depending on the tumor location and the condition of the patients.
Moreover, the insights gathered from tissue biopsy only reflect the biological traits of the sampled tumor tissue. So, in some cases this information cannot represent the entire tumor.
Liquid Biopsy vs Tissue biopsy
Liquid Biopsy is a technology that can detect various genetic changes in cancer cells using the circulating tumor DNA (ctDNA) or circulating tumor cell (CTC), exosome, microRNA, tumor educated platelets. With the right sensitivity and specificity, these can be used for diagnosis, treatment decisions and monitoring of cancers. Among them, the first to be applied clinically was liquid biopsy using ctDNA from the peripheral blood.

How is ctDNA Detected?
Various Methods from Single variant analysis to full length genome analysis techniques are available for ctDNA analysis.
The sensitivity and regions for detection may vary depending on the method chosen.
Among the various techniques available, the GCG-Oncomine Pan-cancer (LBx) Analysis Service utilizes next-generation sequencing (NGS) to analyze ctDNA and report genetic variants.
| Technique | Sensitivity | Optimal Application | |
|---|---|---|---|
| Sanger sequencing |  |
>10% | Tumor tissue |
| Pyrosequencing | 10% | Tumor tissue | |
| Next-generation sequencing | 2% | Tumor tissue | |
| QuantativePCR | 1% | Tumor tissue | |
| ARMS | 0.10% | Tumor tissue | |
| BEAMing, PAP, Digital PCR, TAM-Seq | 0.01% or lower | ctDNA, rare variants in tumor tissue |
Reference: J Clin Oncol. 2014;32(6):579-586
Test Performance
| Reporting Threshold | Analytical Sensitivity | Analytical Specificity | |||
|---|---|---|---|---|---|
| SNVs | 0.1* – 0.5% (2~3 molecules) |
LOD | ≶1% | 92.1-96.9% | 99.9% |
| ≥1% | 100% | ||||
| Indel | 0.1* – 0.5% (2~3 molecules) |
LOD | ≶1% | 92.1-96.9% | 99.9% |
| ≥1% | 100% | ||||
| Fusions | 1.0% (2 molecules) | 100% | 100% | ||
| CNVs | 2.3 – 4.0 copies (gain) | 100% | 100% | ||
| 1.0 copies (loss) | |||||
* Based on cfDNA input of 20ng